Abstract

Background: Patients with refractory NHL experience poor outcomes to currently available therapies. In the SCHOLAR-1 pooled analysis of patients with refractory aggressive NHL, the objective response rate (ORR) was 26% (complete response [CR] rate was 7%), and the median overall survival (OS) was 6.3 months (Crump et al. Blood . In press). The primary analysis of ZUMA-1 demonstrated positive results with an ORR of 82% and a CR rate of 54% after a single infusion of axi-cel. The safety profile was manageable: grade ≥ 3 cytokine release syndrome and neurologic events were generally reversible and reported for 13% and 28%, respectively (Locke et al. AACR 2017. #9986). With a median follow-up of 8.7 months, 44% of patients in ZUMA-1 were in ongoing response. We plan to present the 1-year follow-up of ZUMA-1 to confirm the stability of response following anti-CD19 CAR T cell therapy as previously suggested (Locke et al. Mol Ther . 2016; Kochenderfer et al. Mol Ther . 2017). Additionally, exploratory biomarker analyses were conducted to understand the mechanisms of resistance to anti-CD19 CAR T cell treatment.
Methods: Patients with refractory diffuse large B cell lymphoma, transformed follicular lymphoma, or primary mediastinal large B cell lymphoma were enrolled and dosed per Locke et al. (AACR 2017. #9986). Refractory disease was defined as progressive disease or stable disease as best response to last line of therapy, or relapse ≤ 12 months after autologous stem cell transplant. Patients must have had a prior anti-CD20 antibody and an anthracycline-containing regimen.The primary endpoint was ORR per 2007 International Working Group criteria. Key secondary endpoints included duration of response (DOR), OS, and incidence of adverse events (AEs). A key exploratory endpoint was to investigate the mechanisms of resistance using post-treatment tumor biopsies obtained at time of relapse or progression.
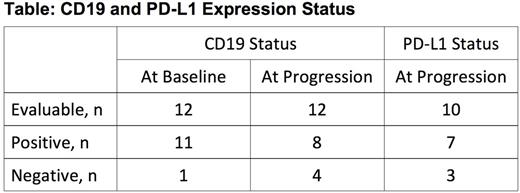
Results: A long-term follow-up analysis will be performed with a data cut-off of August 11, 2017. To date, no patients have been lost to follow-up, and all patients who are alive remain in disease and survival follow-up. Updated DOR and OS will be presented with a minimum follow-up of 1 year and a median follow-up of 15 months. Updated subgroups and associative analyses of efficacy outcomes will be presented. Baseline and post-progression biopsies were evaluable by central review from 12 patients. CD19 and PD-L1 immunohistochemistry results are tabulated. Three of 11 (27%) patients with CD19-positive status at baseline developed CD19-negative disease at time of disease progression. Eight of 10 (80%) patients evaluable for PD-L1 at time of disease progression had PD-L1-positive disease. Of the 8 patients with CD19-positive samples at progression, 5 (63%) demonstrated PD-L1-positive tumor cells. Of the 3 patients with CD19-negative samples at progression, 2 had PD-L1-positive tumor cells.
In addition, post-progression biopsies from 6 separate patients were evaluable by local review, of which 3 (50%) had ≤ 1% CD19 staining. Cumulatively, 17 patients were evaluable for CD19 expression at time of progression by either central or local review, and 6 (35%) had ≤ 1% CD19 expression. Updated results will be presented.
Conclusions: In the ZUMA-1 study, axi-cel demonstrated significant clinical benefit with manageable AEs in patients with no curative treatment options. Additional long-term efficacy, safety, subgroup, and biomarker associative analyses with a median of 15 months of follow-up will be presented. Loss of CD19 and gain of PD-L1 expression in tumors are identified as possible mechanisms of resistance following axi-cel treatment. These results provide insights into development of novel therapeutic strategies to overcome CD19 CAR T resistance and further improve outcomes in these patients.
Drs. Neelapu and Locke contributed equally to this work.
Neelapu: BMS: Research Funding; Poseida: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Locke: Kite Pharma: Consultancy; Cellular Biomedicine Group Inc: Consultancy. Miklos: Genentech: Research Funding; Roche: Research Funding; Adaptive Biotechnologies: Consultancy, Other: Travel expenses; Kite Pharma: Consultancy, Other: Travel expenses, Research Funding; Pharmacyclics, LLC: Consultancy, Other: Travel expenses, Patents & Royalties, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel expenses; Sanofi: Honoraria; Novartis: Research Funding. Jacobsen: Spectrum: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees. Braunschweig: Kite Pharma: Equity Ownership. Oluwole: Kite Pharma: Consultancy. Siddiqi: Juno: Other: Steering committee for JCAR017; Seattle Genetics: Speakers Bureau; Pharmacyclics, an AbbVie Company: Other: Steering committee for ibrutinib, Speakers Bureau. Timmerman: ImmuneGene: Research Funding; Kite Pharma: Research Funding; Seattle Genetics: Consultancy; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Genmab: Consultancy, Equity Ownership. Reagan: Seattle Genetics: Research Funding. Bot: Kite Pharma: Employment, Equity Ownership. Rossi: Kite Pharma: Employment, Equity Ownership. Navale: Kite Pharma: Employment, Equity Ownership. Jiang: Kite Pharma: Employment, Equity Ownership. Aycock: Kite Pharma: Employment, Equity Ownership. Elias: Kite Pharma: Employment, Equity Ownership. Wiezorek: Kite Pharma: Employment, Equity Ownership. Go: Kite Pharma: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal